
Chemistry, 18.09.2019 22:00 shoafmckenzie5263
What volume of water (solute) (d = 1.00 g/ml) should be added to 600. ml of ethanol (solvent, c2h5oh) in order to have a solution that boils at 95.0°c? [for ethanol, kb = 1.22 °c/m, density = 0.789 g/ ml, boiling point = 78.4°c]

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, FloweyFlower
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? a balloon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 22.06.2019 17:30, kiaramccurty
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
What volume of water (solute) (d = 1.00 g/ml) should be added to 600. ml of ethanol (solvent, c2h5oh...
Questions in other subjects:

History, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40



Mathematics, 20.11.2020 18:40

Arts, 20.11.2020 18:40




Mathematics, 20.11.2020 18:40

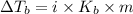
 = elevation in boiling point
= elevation in boiling point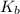 =boiling point constant =
=boiling point constant = 
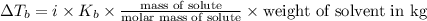


 (1kg=1000g)
(1kg=1000g)






