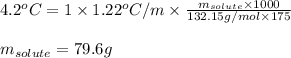Chemistry, 18.09.2019 21:20 tatyanaknight122
Cinnamaldehyde (mm = 132.15 g/mol) is used as a flavoring agent. what mass of cinnamaldehyde must be added to 175 g of ethanol to give a solution whose boiling point is 82.7°c? kb = 1.22°c/m, boiling point of pure ethanol = 78.5°c

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chefdnguyen
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1


Chemistry, 22.06.2019 04:30, anthony4034
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3
You know the right answer?
Cinnamaldehyde (mm = 132.15 g/mol) is used as a flavoring agent. what mass of cinnamaldehyde must be...
Questions in other subjects:










Mathematics, 12.02.2020 02:43

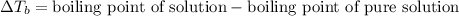
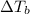 = ? °C
= ? °C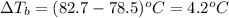
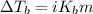
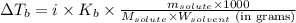
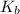 = molal boiling point elevation constant = 1.22°C/m.g
= molal boiling point elevation constant = 1.22°C/m.g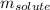 = Given mass of solute (cinnamaldehyde) = ? g
= Given mass of solute (cinnamaldehyde) = ? g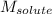 = Molar mass of solute (cinnamaldehyde) = 132.15 g/mol
= Molar mass of solute (cinnamaldehyde) = 132.15 g/mol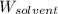 = Mass of solvent (ethanol) = 175 g
= Mass of solvent (ethanol) = 175 g