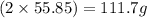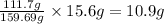Chemistry, 18.09.2019 17:10 michellemonroe012305
A29.7 g sample of iron ore is treated as follows. the iron in the sample is all converted by a series of chemical reactions to fe₂o₃. the mass of fe₂o₃ is measured to be 15.6 g. what was the mass of iron in the sample of ore? answer in units of g.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:00, robert7248
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
A29.7 g sample of iron ore is treated as follows. the iron in the sample is all converted by a serie...
Questions in other subjects:

Chemistry, 20.05.2020 17:59


History, 20.05.2020 17:59

English, 20.05.2020 17:59



Social Studies, 20.05.2020 17:59


Social Studies, 20.05.2020 17:59