
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o2) are carbon dioxide (co2) and water (h2o).
a mass of 16.74 g for an unknown fuel was combusted in a reaction vessel containing an unknown amount of oxygen. at the end of the reaction, there still remained 16.70 g of the fuel as well as 0.0654 g of water and 0.1198 g of carbon dioxide. the oxygen was completely consumed during the reaction.
how many molecules of oxygen gas were initially present in the reaction vessel?
me, i will be very . the topic is limiting reagents and theoretical yields.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2


You know the right answer?
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o...
Questions in other subjects:

Mathematics, 15.11.2020 01:30


Spanish, 15.11.2020 01:30







Biology, 15.11.2020 01:30



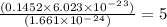 molecules
molecules


