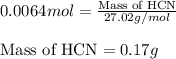Chemistry, 18.09.2019 04:10 potaetoo1997
When potassium cyanide (kcn) reacts with acids, a deadly poisonous gas, hydrogen cyanide (hcn), is given off: kcn(aq) + hcl(aq) → hcn(g) + kcl(aq) if a sample of 0.420 g of kcn is treated with an excess of hcl, calculate the amount of hcn formed in grams.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
You know the right answer?
When potassium cyanide (kcn) reacts with acids, a deadly poisonous gas, hydrogen cyanide (hcn), is g...
Questions in other subjects:




Physics, 27.09.2021 18:00

Mathematics, 27.09.2021 18:00

Mathematics, 27.09.2021 18:00


Physics, 27.09.2021 18:00

Mathematics, 27.09.2021 18:00

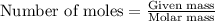 .....(1)
.....(1)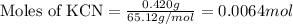
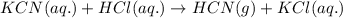
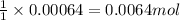 of hydrogen cyanide
of hydrogen cyanide