
Chemistry, 18.09.2019 04:10 lmoleary7466
Asample of ammonium nitrate, weighing 25.2 g, was added to a 150.0 ml container of h2o at 20.0 °c, and then is thoroughly dissolved. the final temperature of the mixture is 8.5 °c. find how much heat (kj) was lost/gained by the solution (surroundings).

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3


Chemistry, 23.06.2019 09:40, 23rwilliamson
Is cutting your nails a physical or chemical change
Answers: 2
You know the right answer?
Asample of ammonium nitrate, weighing 25.2 g, was added to a 150.0 ml container of h2o at 20.0 °c, a...
Questions in other subjects:





Mathematics, 29.05.2020 19:03






 = change in temperature
= change in temperature is
is 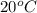 ,
,  is
is  , and specific heat of water is 4.184
, and specific heat of water is 4.184  .
.



