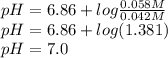Chemistry, 18.09.2019 03:00 tnbankspines
The phosphate buffer system is very important for maintaining the ph of the cytoplasm of all cells. phosphoric acid is a triprotic acid; however, the relevant equilibrium in the biologically useful, neutral range, with a pka of 6.86, is that of dihydrogen phosphate and monohydrogen phosphate ions: h3po4 ⇌ h2po4- + h+ pka = 2.14 h2po4- ⇌ hpo42- + h+ pka = 6.86 hpo42- ⇌ po43- + h+ pka = 12.4 using the henderson-hasselbalch equation, calculate the ph of a solution containing 0.042 m nah2po4 and 0.058 m na2hpo4.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, artemiscrock77041
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 01:00, deidaralove90
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 16:00, esme06quirino
Is a measure of the resistance to flow. a high liquid has a high resistance to flow and flows slowly. the ancients thought everything in the world was made of 4 we now know that there are 94 naturally occurring and scientists have created another 24 i am certain they will create even more. honey flows slowly because it has a high to flowing. a can be separated by physical means because it contains more than one pure substance and 2 pure substances are not chemically bonded to each other. a cannot be separated by physical means. all matter is made up of all elements are with the same number of protons. if it is just a single or many bonded together, if all of them have the same number of protons, it is an element. in a piece of pure iron metal, all the are joined together, that piece of iron metal is called elemental iron. a single of iron is called elemental iron. a mixture has differences from place to place. we might need a microscope to see them or they might be obvious to the unaided eye. there are surfaces separating it into different phases. a mixture is the same everywhere. it is uniform. there are no surfaces separating it into different phases. if different kinds of atoms (different elements) are bonded together by their electrons, it is called a there are physical means of to isolate the different pure substances in a mixture and there are chemical means of to isolate the different elements in a compound. 1. element 2. compound 3. mixture 4. heterogeneous 5. homogeneous 6. pure substance 7. atoms 8. separation 9. viscosity 10. resistance
Answers: 2
You know the right answer?
The phosphate buffer system is very important for maintaining the ph of the cytoplasm of all cells....
Questions in other subjects:


Computers and Technology, 11.01.2021 23:40

Computers and Technology, 11.01.2021 23:40

Biology, 11.01.2021 23:40


History, 11.01.2021 23:40


Mathematics, 11.01.2021 23:40

Biology, 11.01.2021 23:40

English, 11.01.2021 23:40

![pH=pka+log\frac{[A^{-} ]}{[HA]}](/tpl/images/0238/4057/fae8c.png)
![pH=pka+log\frac{[HPO4^{-2} ]}{[H2PO4^{-} ]}](/tpl/images/0238/4057/ec35f.png)