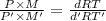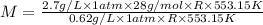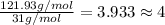Chemistry, 18.09.2019 02:00 davisparker5269
At its boiling point (280°c) and at atmospheric pressure, phosphorus gas has a density of 2.7 g l–1 . under the same conditions, nitrogen gas has a density of 0.62 g l–1 . how many atoms of phosphorus are there in one phosphorus molecule under these conditions?

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:00, Ashleyvasquez2261
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2
You know the right answer?
At its boiling point (280°c) and at atmospheric pressure, phosphorus gas has a density of 2.7 g l–1...
Questions in other subjects:

History, 12.09.2019 21:10

Mathematics, 12.09.2019 21:10

Mathematics, 12.09.2019 21:10





Chemistry, 12.09.2019 21:10

Arts, 12.09.2019 21:10

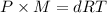 ...(1)
...(1)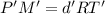
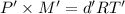 ...(2)
...(2)