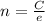Chemistry, 17.09.2019 23:30 alyssa0888
To analyze the experiment used to determine the properties of an electron. in 1909, robert millikan performed an experiment involving tiny, charged drops of oil. the drops were charged because they had picked up extra electrons. millikan was able to measure the charge on each drop in coulombs. here is an example of what his data may have looked like.
drop charge (c)
a 3.20 x 10^{-19}
b 4.80 x 10^{-19}
c 8.00 x 10^{-19}
d 9.60 x 10^{-19}
based on the given data, how many extra electrons did drop c contain?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 23.06.2019 00:00, savyblue1724707
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 00:50, lakhanir2013
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
To analyze the experiment used to determine the properties of an electron. in 1909, robert millikan...
Questions in other subjects:




History, 22.11.2020 07:10

History, 22.11.2020 07:10

Health, 22.11.2020 07:10


Biology, 22.11.2020 07:10