
Amixture of noble gases [helium (mw 4), argon (mw 40), krypton (mw 83.8), and xenon (mw 131.3)] is at a total pressure of 150 kpa, and a temperature of 500 k. the mixture has the following composition in mole fraction: 0.25 helium, 0.25 argon, 0.25 krypton. determine: (a) the mass fraction of helium. (b) the average molecular weight of the mixture. (c) the total molar concentration. (d) the mass density.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, haybaby312oxdjli
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 21.06.2019 22:00, pettygirl13
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 00:00, reeceslife481
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

You know the right answer?
Amixture of noble gases [helium (mw 4), argon (mw 40), krypton (mw 83.8), and xenon (mw 131.3)] is a...
Questions in other subjects:


Mathematics, 23.06.2020 23:01



Mathematics, 23.06.2020 23:01

Mathematics, 23.06.2020 23:01



Mathematics, 23.06.2020 23:01

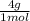 = 1 g of HeliumArgon: 0,25 moles ×
= 1 g of HeliumArgon: 0,25 moles ×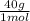 = 10 g of ArgonKrypton: 0,25 moles ×
= 10 g of ArgonKrypton: 0,25 moles ×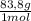 = 20,95 g of krypton Xenon: 0,25 moles ×
= 20,95 g of krypton Xenon: 0,25 moles ×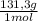 = 32,825 g of Xenon
= 32,825 g of Xenon × 100 = 1,6%
× 100 = 1,6%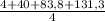 = 64,775 g/mol
= 64,775 g/mol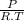 = M
= M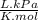
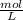 ×
×  ×
× 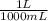 =
=

