
Chemistry, 17.09.2019 21:20 DivineMemes420
You are asked by the king of allegesia to determine if the crown he was given for his birthday is pure gold. the density of pure gold is 19.32 g/ml; the density of water is 0.998 g/ml at 20ºc. there is a container when filled to the mark contains 1900.0 ml. when the container is filled with water to the mark, it has a mass of 2499.5 g. the crown is placed in the container and filled to the mark with water and now weighs 15,113.3 g. the crown has a mass of 13,910.0 g. what is the density of the crown? is the crown pure gold?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2


Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 21:30, sarah192002
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
You are asked by the king of allegesia to determine if the crown he was given for his birthday is pu...
Questions in other subjects:



Physics, 01.04.2020 17:46

World Languages, 01.04.2020 17:46

English, 01.04.2020 17:46

Health, 01.04.2020 17:46



Social Studies, 01.04.2020 17:46

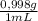 = 1896,2 g of water
= 1896,2 g of water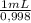 = 601,2 mL
= 601,2 mL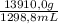 = 10,71 g/mL
= 10,71 g/mL

