
Chemistry, 17.09.2019 21:00 ashvinmsingh
Achemist needs to create a series of standard cu2+(aq)cu2+(aq) solutions for an absorbance experiment. for the first standard, he uses a pipet to transfer 5.005.00 ml of a 2.172.17 m cu2+(aq)cu2+(aq) stock solution to a 500.0500.0 ml volumetric flask, and he adds enough water to dilute to the mark. he then uses a second pipet to transfer 25.0025.00 ml of the second solution to a 100.0100.0 ml volumetric flask, and he adds enough water to dilute to the mark. calculate the concentration of the cu2+(aq)cu2+(aq) solution in the 100.0100.0 ml volumetric flask.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, flowergirly34
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
Achemist needs to create a series of standard cu2+(aq)cu2+(aq) solutions for an absorbance experimen...
Questions in other subjects:







Health, 05.03.2020 10:48



Mathematics, 05.03.2020 10:49

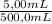 = 0,0217 M Cu²⁺solution
= 0,0217 M Cu²⁺solution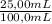 = 5,425×10⁻³ M Cu²⁺ solution
= 5,425×10⁻³ M Cu²⁺ solution

