
Iron reacts with oxygen to produce iron(iii) oxide as represented above. a 75.0 g sample of fe(s)is mixed with 11.5 l of o2(g) at 2.66 atm and 298 k.(a) calculate the number of moles of each of the following before the reaction occurs.(i) fe(s)(ii) o2(g)(b) identify the limiting reactant when the mixture is heated to produce fe2o3. support youranswer with calculations.(c) calculate the number of moles of fe2o3 produced when the reaction proceeds tocompletion.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, clairajogriggsk
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 04:30, akeemedwards12
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
Iron reacts with oxygen to produce iron(iii) oxide as represented above. a 75.0 g sample of fe(s)is...
Questions in other subjects:




Mathematics, 13.05.2021 18:40


Mathematics, 13.05.2021 18:40




Mathematics, 13.05.2021 18:40

 = 1,34 Fe(s) moles
= 1,34 Fe(s) moles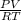 = n
= n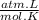
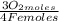 = 1,01 O₂ moles
= 1,01 O₂ moles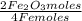 =
= 

