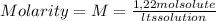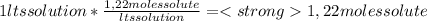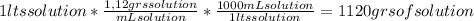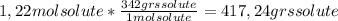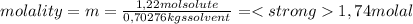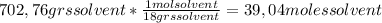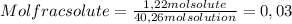Chemistry, 17.09.2019 02:00 romaguera06
A) calculatethe molality, m, of an aqueous solution of 1.22 m sucrose, c12h22o11. the density of the solution is 1.12 g/ml. b) what is the mass percent of sucrose in this solution? c) what is the mole fraction of sucrose in this solution?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1


Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
A) calculatethe molality, m, of an aqueous solution of 1.22 m sucrose, c12h22o11. the density of the...
Questions in other subjects:

Mathematics, 14.12.2020 18:00

English, 14.12.2020 18:00

Spanish, 14.12.2020 18:00

Chemistry, 14.12.2020 18:00

Mathematics, 14.12.2020 18:00




Mathematics, 14.12.2020 18:00