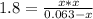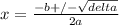Chemistry, 16.09.2019 19:30 gracebuffum
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(g)kc=1.80 at 250 ∘c a 0.157 mol sample of pcl5(g) is injected into an empty 2.50 l reaction vessel held at 250 ∘c. calculate the concentrations of pcl5(g) and pcl3(g) at equilibrium.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

You know the right answer?
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(g)kc=1...
Questions in other subjects:



Mathematics, 04.03.2020 19:42

History, 04.03.2020 19:43




Mathematics, 04.03.2020 19:44

Spanish, 04.03.2020 19:45

![Kc = \frac{[C]^cx[D]^d}{[A]^ax[B]^b}](/tpl/images/0233/9746/d3f86.png)
![Kc = \frac{[PCl3]x[Cl2]}{[PCl5]}](/tpl/images/0233/9746/20f28.png)