

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3
You know the right answer?
Calculate the atomic weight of rubidium, which has two isotopes with the following properties: 85^r...
Questions in other subjects:

Health, 06.04.2021 17:50

English, 06.04.2021 17:50

English, 06.04.2021 17:50


Chemistry, 06.04.2021 17:50




English, 06.04.2021 17:50

Chemistry, 06.04.2021 17:50

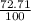 = 61.73 amu
= 61.73 amu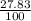 = 24.18 amu
= 24.18 amu

