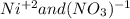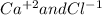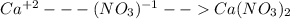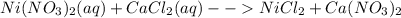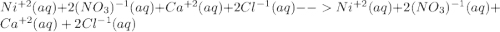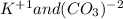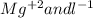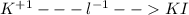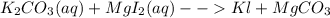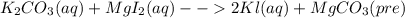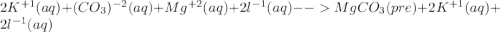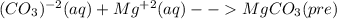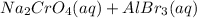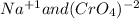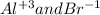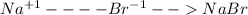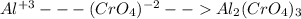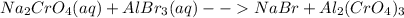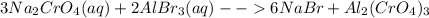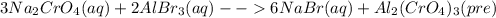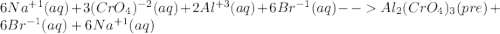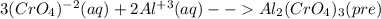Chemistry, 16.09.2019 18:30 trejo282961
For the following reactions, predict the products and write the balanced formula equation, complete ionic equation, and net ionic equation. if no precipitate forms, write "no reaction: " a. hg2(no3)2(aq) + cuso4(aq) b. ni(no3)2(aq) + cacl2(aq) c. k2co3(aq) + mgi2(aq) d. na2cro4(aq) + albr3(aq)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 23.06.2019 05:00, shealynh52
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2

You know the right answer?
For the following reactions, predict the products and write the balanced formula equation, complete...
Questions in other subjects:


Biology, 03.08.2019 17:00



Mathematics, 03.08.2019 17:00


History, 03.08.2019 17:00


Mathematics, 03.08.2019 17:00

History, 03.08.2019 17:00

![Hg^{+1} - - - (SO_{4})^{-2}[/text] the oxidation number will tell you the subscript for each species in the compound. In this case, is Hg2(SO4) [tex]Cu^{+2} - - - (NO_{3})^{-1} - - - Cu(NO_{3})_{2} [/text] So, the products for this reaction will be [tex]Hg_{2} (NO_{3})_{2}(aq) + CuSO_{4}(aq) -- Hg_{2}SO_{4} + Cu(NO_{3})_{2}[/text] After this, we proceed to balance the equation. For this, we check that we have the same number of each element on both sides of the equation. In this case, we can see that we have the same number, so the equation is balanced. Finally, we check the rules of solubility to see if the species are soluble in water or not. In this case sulfates area always soluble except for mercury so Hg2(SO4) precipitates in the solution (pre). Nitrates are always soluble so Cu(NO3)2 is soluble (aq) [tex] Hg_{2}(NO_{3})_{2}(aq) + CuSO_{4}(aq) - - Hg_{2}SO_{4} (pre) + Cu(NO_{3})_{2}(aq)](/tpl/images/0233/8166/40fae.png)
![2Hg^{+1}(aq) + 2(NO_{3})^{-1}(aq) + (SO_{4})^{-2}(aq) + Cu^{+2}(aq) -- Hg_{2}SO_{4} (pre)+ Cu^{+2}(aq) + (NO_{3})^{-1}(aq) [/text] To get net ionic equation we take away the ions that did not participate in the reactions. In other words the ones that are the same on both sides in the equation. In this case we see that [tex] Cu^{+2}(aq) and (NO_{3})^{-1}(aq) [/text] are the same on both sides so those ions are not include in the net ionic equation. This is: [tex] 2Hg^{+1}(aq) + (SO_{4})^{-2}(aq) -- Hg_{2}SO_{4} (pre) [/text] B [tex] Ni(NO_{3})_{2}(aq) + CaCl_{2}(aq)](/tpl/images/0233/8166/58e48.png)