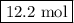Chemistry, 14.09.2019 11:30 TyleenValdez975
Calculating and using the molar mass of heterodiatomic the chemical formula for lithium fluoride is lif a chemist measured the amount of lithium fluoride produced during an experiment. she finds that 317. g of lithium fluoride is produced. calculate the number of moles of lithium fluoride produced. round your answer to 3 significant digits. mol x explanation check 2019 mcgrawh terms of education all righes reserved

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:50, limelight11
Which statement describes how phase changes can be diagrammed as a substance is heated? the phase is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the phase is on the x-axis. the time is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the time is on the x-axis.
Answers: 1


Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
You know the right answer?
Calculating and using the molar mass of heterodiatomic the chemical formula for lithium fluoride is...
Questions in other subjects:

Mathematics, 31.07.2019 14:10








Mathematics, 31.07.2019 14:10

English, 31.07.2019 14:10