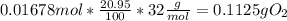Chemistry, 14.09.2019 11:30 loredobrooke9929
At 14,000 ft elevation the air pressure drops to 0.59 atm. assume you take a 1l sample of air at this altitude and compare it to 1 l of air taken at sea level. how much less o2 (in g) is available in 1 l of air at 14,000 ft (assume temperature of 298 k and that relative gas percentages are constant in both locations).

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, allisondelv67
Activity two: just lemons, inc. production here's a one-batch sample of just lemons lemonade production. determine the percent yield and amount of leftover ingredients for lemonade production and place your answers in the data chart. hint: complete stoichiometry calculations for each ingredient to determine the theoretical yield. complete a limiting reactant-to-excess reactant calculation for both excess ingredients. water sugar lemon juice lemonade percent yield leftover ingredients 946.36 g 196.86 g 193.37 g 2050.25 g just lemons lemonade recipe equation: 2 water + sugar + lemon juice = 4 lemonade mole conversion factors: 1 mole of water = 1 cup = 236.59 g 1 mole of sugar = 1 cup = 225 g 1 mole of lemon juice = 1 cup = 257.83 g 1 mole of lemonade = 1 cup = 719.42 g
Answers: 2

Chemistry, 22.06.2019 06:40, CylieTbh
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 16:00, sassy11111515
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
At 14,000 ft elevation the air pressure drops to 0.59 atm. assume you take a 1l sample of air at thi...
Questions in other subjects:

English, 16.01.2020 10:31

Biology, 16.01.2020 10:31





Mathematics, 16.01.2020 10:31