Chemistry, 14.09.2019 11:30 tanyadeewill
7. for the system pcls(g) → pc13(g) + cl2(g) kis 26 at 300°c. in a 5.0-l flask, a gaseous mixture consists of all three gases with partial pressure as follows: ppcis = 0.012 atm, pc2=0.45 atm, ppci3 -0.90 atm. a) is the mixture at equilibrium? explain. b) if it is not at equilibrium, which way will the system shift to establish equilibrium?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 13:50, hannahmyung1113
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 17:30, shookiegriffin
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1
You know the right answer?
7. for the system pcls(g) → pc13(g) + cl2(g) kis 26 at 300°c. in a 5.0-l flask, a gaseous mixture co...
Questions in other subjects:

English, 24.02.2020 21:00




Mathematics, 24.02.2020 21:00




Mathematics, 24.02.2020 21:00

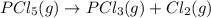
![Reaction\ quotient (Q) = \frac{[p_{PCl_3}]\times [p_{Cl_2}]}{[p_{PCl_5}]}](/tpl/images/0231/3431/4e28d.png)
![[p_{PCl_5}] = 0.012 atm](/tpl/images/0231/3431/6af84.png)
![[p_{PCl_3}]= 0.90 atm](/tpl/images/0231/3431/1d822.png)
![[p_{Cl_2}]= 0.45 atm](/tpl/images/0231/3431/b9835.png)