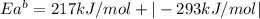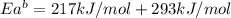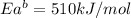Chemistry, 14.09.2019 11:10 joelpimentel
The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 217 kj/mol and the change in enthalpy for the reaction is δh = -293 kj/mol .
what is the activation energy for the reverse reaction?
enter your answer numerically and in terms of kj/mol.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 15:30, ricardotavarez6
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 217 kj/mol and th...
Questions in other subjects:


Computers and Technology, 10.07.2019 09:30

History, 10.07.2019 09:30

Mathematics, 10.07.2019 09:30




Computers and Technology, 10.07.2019 09:30

Mathematics, 10.07.2019 09:30

Physics, 10.07.2019 09:30

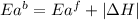
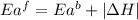
 = activation energy for forward reaction
= activation energy for forward reaction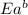 = activation energy for backward reaction
= activation energy for backward reaction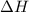 = change in enthalpy of reaction
= change in enthalpy of reaction