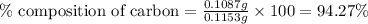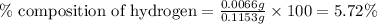Chemistry, 14.09.2019 09:30 bagofmud8339
A0.1153-gram sample of a pure hydrocarbon was burned in a c-h combustion train to produce 0.3986 gram of co2and 0.0578 gram of h2o. determine the masses of c and h in the sample and the percentages of these elements in this hydrocarbon.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1


Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
A0.1153-gram sample of a pure hydrocarbon was burned in a c-h combustion train to produce 0.3986 gra...
Questions in other subjects:

Mathematics, 16.04.2020 03:40



History, 16.04.2020 03:40






Arts, 16.04.2020 03:40

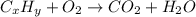
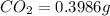
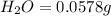
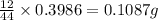 of carbon will be contained.
of carbon will be contained.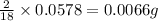 of hydrogen will be contained.
of hydrogen will be contained.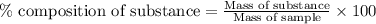 ......(1)
......(1)