Chemistry, 14.09.2019 09:30 litwork5885
Hexane is a common laboratory solvent and a component of petrol. its combustion reaction is as follows: 2 c6h14 (9) + 19 02(9) +12 co2(g) + 14 h20(1) arh=-8326 kj mol-1 use the reaction enthalpy value to calculate the heat in (kj) released by combustion of 0.121 mol of hexane. (give your answer as a positive number to at least three significant figures and do not include the units in the answer box)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
Hexane is a common laboratory solvent and a component of petrol. its combustion reaction is as follo...
Questions in other subjects:

Mathematics, 11.04.2020 17:14

Mathematics, 11.04.2020 17:15

Mathematics, 11.04.2020 17:16

Mathematics, 11.04.2020 17:16




History, 11.04.2020 17:17


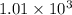
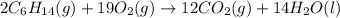
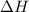 = -8326 kJ/mol
= -8326 kJ/mol