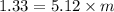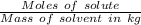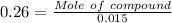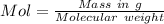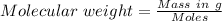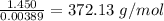A1.450 g sample of an unknown organic compound , x, is dissolved in 15.0 g of toluene
( c7h8 =...

Chemistry, 14.09.2019 09:10 allendm5166
A1.450 g sample of an unknown organic compound , x, is dissolved in 15.0 g of toluene
( c7h8 = 92 g/mol) and the freezing point is lowered by 1.33 oc. what is the molecular weight
of the organic compound? (kf = 5.12 oc/m).

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, kkmonsterhigh18
The diagram below shows a cell placed in a solution. a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution. only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it. it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 11.04.2020 06:55

Spanish, 11.04.2020 06:55


Mathematics, 11.04.2020 06:55



English, 11.04.2020 06:55

Mathematics, 11.04.2020 06:55


Chemistry, 11.04.2020 06:55

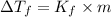
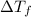 = Depression in freezing point
= Depression in freezing point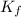 = Molal depression constant
= Molal depression constant