
Chemistry, 14.09.2019 08:30 postorivofarms
Methods: part a: preparation of buffers make two buffers starting with solid material, which is the most common way to make buffers. you will be given a desired ph, and your task is to prepare 100 ml of two appropriate buffers at a concentration of 0.10 m. one of the buffers will be a phosphate buffer (ph 7.0) and the other will be a tris buffer (ph 8.0). 1. calculate the weight of the buffer you would need to make 100 ml of a 0.10 m solution. weigh out the correct amount and dissolve in 50 ml water. you will need the following compound molecular weights: na2hpo4 (141.96 g/mol), nah2po4 (119.96 g/mol), and tris base (121.1 g/mol).

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 17:50, mytymikey123
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
Methods: part a: preparation of buffers make two buffers starting with solid material, which is th...
Questions in other subjects:


Health, 24.04.2021 04:20

Mathematics, 24.04.2021 04:20



English, 24.04.2021 04:20

Mathematics, 24.04.2021 04:20


History, 24.04.2021 04:20

Spanish, 24.04.2021 04:20

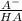
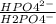
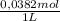 ×
× 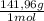 = 0,542 g of Na₂HPO₄
= 0,542 g of Na₂HPO₄ 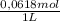 ×
× 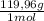 = 0,741 g of NaH₂PO₄
= 0,741 g of NaH₂PO₄ 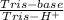
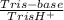 (3)
(3)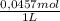 ×
× 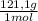 = 0,553 g of Tris-base
= 0,553 g of Tris-base 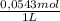 ×
× 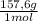 = 0,856 g of Tris-HCl
= 0,856 g of Tris-HCl

