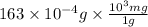Chemistry, 14.09.2019 08:30 mahmudabiazp3ekot
The vapor pressure of water is 28.3 mm hg at 28 °c. what mass of water vapor, in mg, would be present in a vapor volume of 600. ml at 28°c? selected b. 16.3 correct answer b. 16.3

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1



Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
The vapor pressure of water is 28.3 mm hg at 28 °c. what mass of water vapor, in mg, would be presen...
Questions in other subjects:




Biology, 09.07.2019 10:00


Mathematics, 09.07.2019 10:00




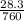 , V = 600 mL =
, V = 600 mL = 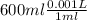 = 0.6 L
= 0.6 L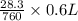 =
= 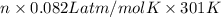
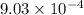 mol
mol 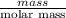
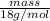
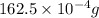
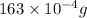 (approx)
(approx)