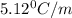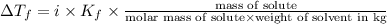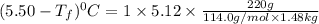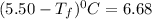Chemistry, 14.09.2019 08:30 lineaeriksen
Calculate the freezing point of a solution made from 220g of octane (c hua), molar mass = 114,0 gmol dissolved in 1480 g of benzene. benzene freezes at 5.50"c and its kvalue is 5.12c/m. -1.16°c 0.98°c 666"c 12 2°c 5.49°c 10 12 am a a 2019 backspace yuo pill но кl

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hannacarroll2539
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1


Chemistry, 22.06.2019 00:30, natalie1755
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1
You know the right answer?
Calculate the freezing point of a solution made from 220g of octane (c hua), molar mass = 114,0 gmol...
Questions in other subjects:

Biology, 29.01.2020 03:46


Mathematics, 29.01.2020 03:46


Social Studies, 29.01.2020 03:46

Biology, 29.01.2020 03:46

English, 29.01.2020 03:46


Spanish, 29.01.2020 03:46

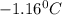
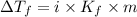
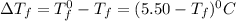 = Depression in freezing point
= Depression in freezing point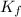 = freezing point constant =
= freezing point constant =