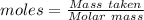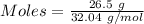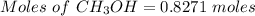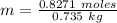Chemistry, 14.09.2019 08:30 icantspeakengles
1826.5g of methanol (ch3oh), molar mass = 32.0 g/mol is added to 735 g of water, what is the molality of the methane 0.0348 m 1.13m 2.03 m 3.61 m 36.1 m navigator f10 delete backspace

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, ggdvj9gggsc
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 05:10, citlalli30
Name a brittle metal , which is used to galvanize iron
Answers: 1
You know the right answer?
1826.5g of methanol (ch3oh), molar mass = 32.0 g/mol is added to 735 g of water, what is the molalit...
Questions in other subjects:









Mathematics, 03.03.2020 03:34

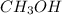 = 26.5 g
= 26.5 g