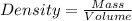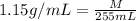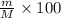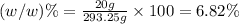Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, dinosaur10
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2


Chemistry, 23.06.2019 07:00, jaydenboi604
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1

Chemistry, 23.06.2019 15:00, jadentdaniels
Solve this problem using the appropriate law. (remember that ) what is the pressure of 1.9 mols of nitrogen gas in a 9.45 l tank and at a temperature of 228 k?
Answers: 1
You know the right answer?
Asolution is prepared by dissolving 20 g naoh (fw = 400 g/mol) to 255 ml of solution. if the density...
Questions in other subjects:


Mathematics, 23.10.2020 14:00



Social Studies, 23.10.2020 14:00


Mathematics, 23.10.2020 14:00



Mathematics, 23.10.2020 14:00