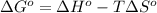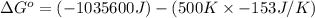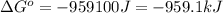Chemistry, 14.09.2019 08:20 87haymaker
Use the given data at 500 k to calculate δg°for the reaction
2h2s(g) + 3o2(g) → 2h2o(g) + 2so2(g)
substance h2s(g) o2(g) h2o(g) so2(g)
δh°f(kj/mol) -21 0 -242 -296.8
s°(j/k·mol) 206 205 189 248

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 23.06.2019 01:30, kcarstensen59070
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
Use the given data at 500 k to calculate δg°for the reaction
2h2s(g) + 3o2(g) → 2h2o(g) + 2so2...
2h2s(g) + 3o2(g) → 2h2o(g) + 2so2...
Questions in other subjects:

Mathematics, 02.12.2020 02:30


English, 02.12.2020 02:30



Mathematics, 02.12.2020 02:30



Arts, 02.12.2020 02:30

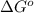 for the reaction is -959.1 kJ
for the reaction is -959.1 kJ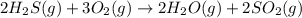
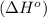 .
.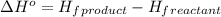
![\Delta H^o=[n_{H_2O}\times \Delta H_f^0_{(H_2O)}+n_{SO_2}\times \Delta H_f^0_{(SO_2)}]-[n_{H_2S}\times \Delta H_f^0_{(H_2S)}+n_{O_2}\times \Delta H_f^0_{(O_2)}]](/tpl/images/0231/1095/c2362.png)
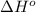 = enthalpy of reaction = ?
= enthalpy of reaction = ?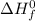 = standard enthalpy of formation
= standard enthalpy of formation![\Delta H^o=[2mole\times (-242kJ/mol)+2mole\times (-296.8kJ/mol)}]-[2mole\times (-21kJ/mol)+3mole\times (0kJ/mol)]](/tpl/images/0231/1095/0563e.png)
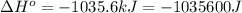
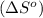 .
.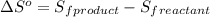
![\Delta S^o=[n_{H_2O}\times \Delta S_f^0_{(H_2O)}+n_{SO_2}\times \Delta S_f^0_{(SO_2)}]-[n_{H_2S}\times \Delta S_f^0_{(H_2S)}+n_{O_2}\times \Delta S_f^0_{(O_2)}]](/tpl/images/0231/1095/f7046.png)
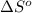 = entropy of reaction = ?
= entropy of reaction = ?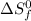 = standard entropy of formation
= standard entropy of formation![\Delta S^o=[2mole\times (189J/K.mol)+2mole\times (248J/K.mol)}]-[2mole\times (206J/K.mol)+3mole\times (205J/K.mol)]](/tpl/images/0231/1095/0797b.png)
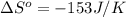
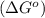 .
.