
Chemistry, 14.09.2019 07:10 Ididntwanttomakethis
Consider the titration of 100 ml of 0.200 m hcho, with 1.00 m naoh. the pk, of hcho2 is 3.75. a) what is the ph before any naoh is added? b) what is the ph after 5.00 ml of naoh are added? c) after 10 ml of naoh are added? d) what is the ph when 20 ml of naoh have been added? what is this point in the titration called?

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
Consider the titration of 100 ml of 0.200 m hcho, with 1.00 m naoh. the pk, of hcho2 is 3.75. a) wha...
Questions in other subjects:

Mathematics, 25.01.2021 18:40

French, 25.01.2021 18:40

Mathematics, 25.01.2021 18:40

Arts, 25.01.2021 18:40

Mathematics, 25.01.2021 18:40

English, 25.01.2021 18:40

Mathematics, 25.01.2021 18:40


Mathematics, 25.01.2021 18:40

![\frac{[x][x] }{[0,200-x]}](/tpl/images/0231/0228/4f77c.png)
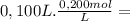 = 2,0x10⁻² mol
= 2,0x10⁻² mol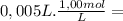 = 5,0x10⁻³ mol
= 5,0x10⁻³ mol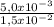
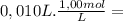 = 1,0x10⁻² mol
= 1,0x10⁻² mol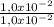
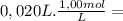 = 2,0x10⁻² mol
= 2,0x10⁻² mol![\frac{[x][x] }{[0,01667-x]}](/tpl/images/0231/0228/2af99.png)


