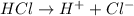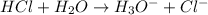Chemistry, 14.09.2019 06:30 theresamarieuehling2
Define the following: bronsted-lowry acid - lewis acid- strong acid - (5 points) problem 6: consider the following acid base reaction hci + h20 → h30+ + cl- a) is this a strong acid? b) clearly label the acid, base, conjugate acid and conjugate base. (5 points)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, goodygoodgirlygirl
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 11:00, peternice2956
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1


Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
Define the following: bronsted-lowry acid - lewis acid- strong acid - (5 points) problem 6: consid...
Questions in other subjects:


Biology, 23.07.2019 08:00

Mathematics, 23.07.2019 08:00

Spanish, 23.07.2019 08:00


English, 23.07.2019 08:00


History, 23.07.2019 08:00

Chemistry, 23.07.2019 08:00

 is a strong acid.
is a strong acid.  , base =
, base = 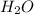 , conjugate acid =
, conjugate acid = 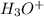
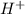 ions.
ions.