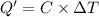In a constant
volume bomb calorimeter, the combustion of 0.6654 gof an organic
compound...

Chemistry, 14.09.2019 05:20 aedmund1225
In a constant
volume bomb calorimeter, the combustion of 0.6654 gof an organic
compound with a molecular mass of 46.07 amu causesthe temperature
in the calorimeter to rise from 25.000oc to 30.589
oc. the total heat capacity ofthe calorimeter and all
its contents is 3576 joc-1. what is
the energy of combustion ofthe organic compound,
du/ kj
mol-1?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:40, hardwick744
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 03:50, sgslayerkingminecraf
Which of the following statements about acidic water is true? a. acid has no effect on the h, o molecules. b. the solution contains a larger number of oh ions than h, o ions. c. the solution contains a larger number of h, o ions than qh ions. d. the solution contains an equal number of h, o ions and oh ions. none of the above e.
Answers: 1
You know the right answer?
Questions in other subjects:




History, 24.01.2020 00:31



Mathematics, 24.01.2020 00:31

Physics, 24.01.2020 00:31

English, 24.01.2020 00:31