
Chemistry, 14.09.2019 05:20 tayfaulk123
Acylinder with a movable piston records a volume of 12.6l when 3.0 mol of oxygen is added. the gas in the cylinder has a pressure of 5.83 atm. the cylinder develops a leak and the volume of gas is now recorded to be 12.1 l at the same pressure. how many moles of oxygen are lost?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1


Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 20:10, sarahalexa19
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
Acylinder with a movable piston records a volume of 12.6l when 3.0 mol of oxygen is added. the gas i...
Questions in other subjects:

Geography, 04.02.2021 23:50



Mathematics, 04.02.2021 23:50

History, 04.02.2021 23:50

English, 04.02.2021 23:50


Mathematics, 04.02.2021 23:50

Biology, 04.02.2021 23:50

History, 04.02.2021 23:50

 (At constant temperature and pressure)
(At constant temperature and pressure)
 = initial volume of oxygen gas = 12.6 L
= initial volume of oxygen gas = 12.6 L = final volume of oxygen gas = 12.1 L
= final volume of oxygen gas = 12.1 L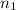 = initial moles of oxygen gas = 3.0 mole
= initial moles of oxygen gas = 3.0 mole = final moles of oxygen gas = ?
= final moles of oxygen gas = ?



