
Chemistry, 25.12.2019 20:31 tommylopez22713
Consider the following reversible, exothermic chemical reaction: 2c2h2(g) + 5o2(g) ↔4co2(g) + 2h2o(g)
all but one of the conditions will cause equilibrium to shift right and produce more of the products. which change will cause the reaction to shift to the left?
a) adding oxygen
b) adding co2
c) increasing the pressure
d) decreasing the temperature

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 23:00, Mw3spartan17
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
Consider the following reversible, exothermic chemical reaction: 2c2h2(g) + 5o2(g) ↔4co2(g) + 2h2o(...
Questions in other subjects:

Social Studies, 16.11.2020 21:50




World Languages, 16.11.2020 21:50

Physics, 16.11.2020 21:50


English, 16.11.2020 21:50

Chemistry, 16.11.2020 21:50

Law, 16.11.2020 21:50

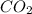
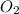 is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of
is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of 

