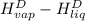Chemistry, 13.09.2019 23:20 SkyeShadow525
The halpy of vaporization of h2o at 1 atm and 100 c is 2259 kj/kg. the heat capacity of liquid water is 4.19 kj/kg. c, and the heat capacity of water vapor is 1.9 kj/kg-c. h20 at 10 bar boils at 179.9 c. what is the enthalpy of vaporization of h20 at 10 bar? you can neglect the effect of pressure. e 2076 kj/kg e 1924 kj/kg e 2259 kj/kg 2442 kj/kg 2594 kj/kg none of the above

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, girlwholikesanime
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 21.06.2019 16:30, kitykay2776
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1
You know the right answer?
The halpy of vaporization of h2o at 1 atm and 100 c is 2259 kj/kg. the heat capacity of liquid water...
Questions in other subjects:




Mathematics, 31.08.2020 01:01



Mathematics, 31.08.2020 01:01

Mathematics, 31.08.2020 01:01


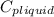 = 4.19
= 4.19 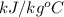
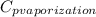 = 1.9
= 1.9 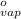 ) at 1 atm and
) at 1 atm and 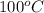 is 2259 kJ/kg
is 2259 kJ/kg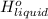 = 0
= 0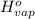 =
= 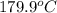 and effect of pressure is not considered. Hence, enthalpy of liquid water at 10 bar and
and effect of pressure is not considered. Hence, enthalpy of liquid water at 10 bar and 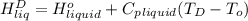
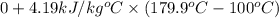
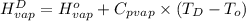
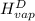 =
= 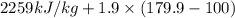
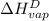 =
=