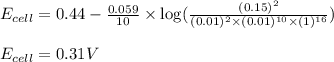Chemistry, 13.09.2019 23:20 michelle230
Calculate the cell potential for a cell operating with the following reaction at 25 degrees celsius, in which [mno4^1-] = .01m, [br^1-] = .01m, [mn^2+] = .15m, and [h^1+] = 1m. the reaction is 2 mno4^1-(aq) + 10 br^1-(aq) + 16 h^1+(aq) --> 2 mn^2+(aq) + 5 br2(l) + 8 h2o(l)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1


Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 12:00, 1963038660
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1
You know the right answer?
Calculate the cell potential for a cell operating with the following reaction at 25 degrees celsius,...
Questions in other subjects:


Mathematics, 25.04.2020 01:01


Mathematics, 25.04.2020 01:01

Physics, 25.04.2020 01:01

History, 25.04.2020 01:01


Mathematics, 25.04.2020 01:01

Mathematics, 25.04.2020 01:01

English, 25.04.2020 01:01

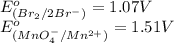
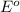 potential will always get reduced and will undergo reduction reaction. Here,
potential will always get reduced and will undergo reduction reaction. Here, 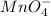 will undergo reduction reaction will get reduced. And, bromine will get oxidized.
will undergo reduction reaction will get reduced. And, bromine will get oxidized.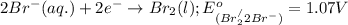 ( × 5)
( × 5)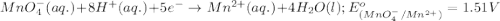 ( × 2)
( × 2)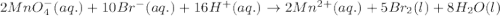
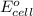 of the reaction, we use the equation:
of the reaction, we use the equation: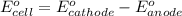
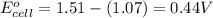
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Mn^{2+}]^2}{[MnO_4^{-}]^2\times [Br^-]^{10}\times [H^+]^{16}}](/tpl/images/0230/4138/86671.png)
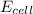 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V![[H^{+}]=1M](/tpl/images/0230/4138/c7b74.png)
![[Mn^{2+}]=0.15M](/tpl/images/0230/4138/f060a.png)
![[MnO_4^{-}]=0.01M](/tpl/images/0230/4138/b97e7.png)
![[Br^{-}]=0.01M](/tpl/images/0230/4138/bb1d7.png)