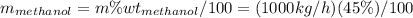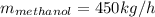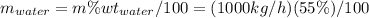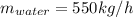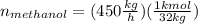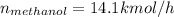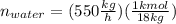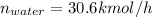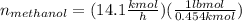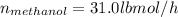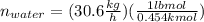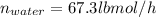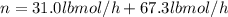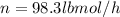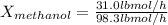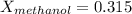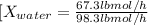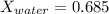Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 23.06.2019 01:20, hflores0001
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 04:31, diamondscott9297
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1

Chemistry, 23.06.2019 06:30, kaitlynk0
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1
You know the right answer?
Conversion of mass to moles a continuous feed to a separation unit is 1,000 kg/h of 45 wt% methanol...
Questions in other subjects:

Chemistry, 28.03.2020 01:56

Mathematics, 28.03.2020 01:56






History, 28.03.2020 01:56


Chemistry, 28.03.2020 01:56