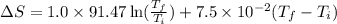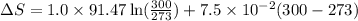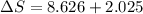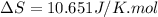in the range 240k to 330k is given

Chemistry, 13.09.2019 23:10 mariaaalopezz
The heat capacity of chloroform (trichloromethane, chcl3)
in the range 240k to 330k is given
bycpm/(jk-1mol-1) = 91.47
+7.5x10-2(t/k). in a particular experiment,
1.0molchcl3 is heated from 273k to 300k. calculate the
changein molar entropy of the sample.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:10, CauseWhyNot6235
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

You know the right answer?
The heat capacity of chloroform (trichloromethane, chcl3)
in the range 240k to 330k is given
in the range 240k to 330k is given
Questions in other subjects:


Mathematics, 16.09.2021 02:40

History, 16.09.2021 02:40




Chemistry, 16.09.2021 02:40

Mathematics, 16.09.2021 02:40


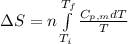
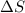 = change in molar entropy
= change in molar entropy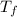 = final temperature = 300 K
= final temperature = 300 K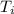 = initial temperature = 273 K
= initial temperature = 273 K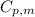 = heat capacity of chloroform =
= heat capacity of chloroform = 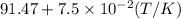
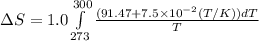
![\Delta S=1.0\times [91.47\ln T+7.5\times 10^{-2}T]^{300}_{273}](/tpl/images/0230/3981/c380e.png)