
To find the formula of a compound composed of iron and carbon monoxide, fex(co)y, the compound is burned in pure oxygen, an reaction that proceeds according to the following unbalanced equation.
fex(co)y + o2 --> fe2o3 + co2
if you burn 1.959 g. of fex(co)y and obtain 0.799 g. of fe2o3 and 2.200 g. of co2, what is the empirical formula of fex(co)y?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, akeemedwards12
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 23:00, lilsnsbsbs
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

You know the right answer?
To find the formula of a compound composed of iron and carbon monoxide, fex(co)y, the compound is bu...
Questions in other subjects:


History, 04.09.2020 01:01


English, 04.09.2020 01:01

Mathematics, 04.09.2020 01:01





Health, 04.09.2020 01:01

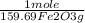 = 5,00×10⁻³ Fe₂O₃ moles
= 5,00×10⁻³ Fe₂O₃ moles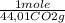 = 5,00×10⁻²CO₂ moles
= 5,00×10⁻²CO₂ moles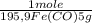 = 1,00×10⁻² Fe(CO)₅ moles
= 1,00×10⁻² Fe(CO)₅ moles

