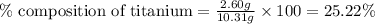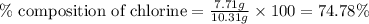Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:20, kekecantonxox121
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2

Chemistry, 22.06.2019 21:30, liamgreene90
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
A2.60 g sample of titanium metal chemically combines
withchlorine gas to form 10.31g of a tita...
withchlorine gas to form 10.31g of a tita...
Questions in other subjects:

Mathematics, 05.09.2020 19:01


English, 05.09.2020 19:01




World Languages, 05.09.2020 19:01



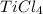
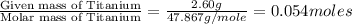
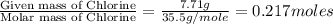
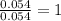
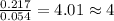
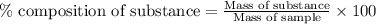 .......(1)
.......(1)