
Chemistry, 13.09.2019 22:30 sosick90501
Question: dimethylhydrazine, the
fuel used in the apollo lunar descentmodule, has a molar mass of
60.10 g/mol. it is made up of carbon, hydrogen, and nitrogen atoms.
the combustion of 2.859g of the fuelin excess oxygen yields 4.190g
of carbon dioxideand 3.428g ofwater. what are the simplest and
molecular formulas fordimethylhydrazine?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1


Chemistry, 22.06.2019 23:00, genyjoannerubiera
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 01:30, kenldykido2300
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
Question: dimethylhydrazine, the
fuel used in the apollo lunar descentmodule, has a molar mass...
fuel used in the apollo lunar descentmodule, has a molar mass...
Questions in other subjects:










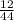 x 4.19g = 1.14g
x 4.19g = 1.14g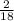 x 3.428g = 0.38g
x 3.428g = 0.38g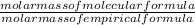 =
= 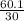 = 2
= 2


