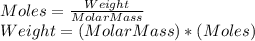Chemistry, 13.09.2019 21:30 ladybuggirl400
Amixture of gases is analyzed and found to have the following volume composition: co2 12.0%, co 6.0, ch4 27.3, h2 9.9, and n2 44.8. how much will 38 [mol] of this mixture weigh, in [kg]?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:10, hadellolo8839
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
You know the right answer?
Amixture of gases is analyzed and found to have the following volume composition: co2 12.0%, co 6.0...
Questions in other subjects:

English, 12.11.2020 03:20

Mathematics, 12.11.2020 03:20

Mathematics, 12.11.2020 03:20



Engineering, 12.11.2020 03:20


History, 12.11.2020 03:20


Mathematics, 12.11.2020 03:20