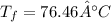Final temperature in heating applesauce. a mixture of 454 kg of applesauce at 10°c is heated in a heat exchanger by adding 121300 kj. calculate the outlet tem perature of the applesauce. (hint: in appendix a.4, a heat capacity for applesauce is given at 32.8c. assume that this is constant and use this as the average com)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, matt16913
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
Final temperature in heating applesauce. a mixture of 454 kg of applesauce at 10°c is heated in a he...
Questions in other subjects:





Computers and Technology, 01.09.2019 02:30



History, 01.09.2019 02:30

Social Studies, 01.09.2019 02:30