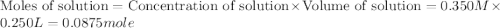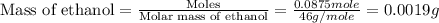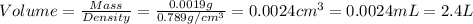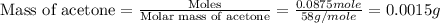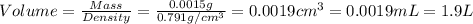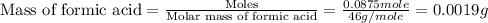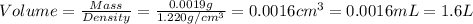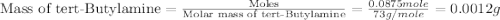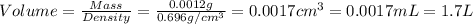Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:30, sbush1412
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u. s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 22:00, jespinozagarcia805
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a. rectant b. product c. supernate
Answers: 3
You know the right answer?
Given the densities of the following pure liquids, what volume of each is necessary to make 250 ml o...
Questions in other subjects:


Mathematics, 27.09.2019 10:00

Arts, 27.09.2019 10:00

History, 27.09.2019 10:00


History, 27.09.2019 10:00

Biology, 27.09.2019 10:00