
Chemistry, 13.09.2019 21:20 angel10999
Khoisan salts is the number 1 producer of salts in sa for both local and international markets. 500 kg of kcl is dissolved in sufficient water to make a saturated solution at 370 k. at 370 kthe solubility of kcl is 42 mass %. the solution is cooled to 320 k and the solubility is 31,5 mass % it is assumed that no water is evaporated. 2.1. determine the amount of water is added to the 500 kg of kcl to produce the required saturated solution at 370 k. (3) 2.2. determine the mass of kcl crystals formed after the cooling process to a temperature of 320 k. (use the formula method)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, lisbet123085
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 22:30, itsmaddierae11
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
Khoisan salts is the number 1 producer of salts in sa for both local and international markets. 500...
Questions in other subjects:

Mathematics, 25.06.2019 14:40


Chemistry, 25.06.2019 14:40

English, 25.06.2019 14:40

Mathematics, 25.06.2019 14:40

Mathematics, 25.06.2019 14:40




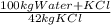 = 1190 kg of water +KCl
= 1190 kg of water +KCl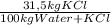 = 375 kg
= 375 kg

