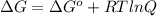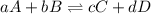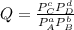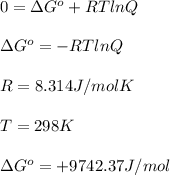At 25 ∘c25 ∘c, the equilibrium partial pressures for the reaction 3a(g)+2b(g)↽−−⇀c(g)+2d(g) 3a(g)+2b(g)↽−−⇀c(g)+2d(g) were found to be pa=5.84pa=5.84 atm, pb=4.47pb=4.47 atm, pc=4.17pc=4.17 atm, and pd=4.32pd=4.32 atm. what is the standard change in gibbs free energy of this reaction at 25 ∘c25 ∘c ?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, kyllow5644
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 06:30, fshane7705
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1

Chemistry, 23.06.2019 08:10, andrewrangel63
An experiment is conducted to see if cats preferred skim milk or 2% milk. a cup of skim milkwas put out for 5 kittens and then measured how much the kittens drank over the course of aday. following a cup of 2% milk was purout for the skittens and then masured how much thekittens drank over the course of a day. the same kittens were used and the milk was served atthe same temperature. it was discovered that the cats liked the 2% milk more than the skimmilk. what is the dependent variable in this experiment?
Answers: 1
You know the right answer?
At 25 ∘c25 ∘c, the equilibrium partial pressures for the reaction 3a(g)+2b(g)↽−−⇀c(g)+2d(g) 3a(g)+2b...
Questions in other subjects:



Chemistry, 09.10.2020 16:01

Mathematics, 09.10.2020 16:01

Spanish, 09.10.2020 16:01




Physics, 09.10.2020 16:01