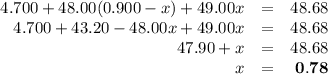Chemistry, 12.09.2019 21:30 SuperWoman9172
Ahypothetical element has an atomic weight of 48.68 amu. it consists of three isotopes having masses of 47.00 amu, 48.00 amu, and 49.00 amu. the lightest-weight isotope has a natural abundance of 10.0%. what is the percent abundance of the heaviest isotope?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:50, stephaniero6
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3


Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 23.06.2019 07:30, danielahumajova6
How do you interpret a chromagram for what mixtures contain?
Answers: 1
You know the right answer?
Ahypothetical element has an atomic weight of 48.68 amu. it consists of three isotopes having masses...
Questions in other subjects:





Mathematics, 16.07.2019 06:30

Chemistry, 16.07.2019 06:30

Mathematics, 16.07.2019 06:30

Mathematics, 16.07.2019 06:30

English, 16.07.2019 06:30

Mathematics, 16.07.2019 06:30