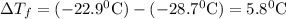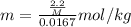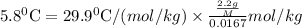Asolution was prepared by dissolving 2.2 g of an unknown solute in 16.7 g of ccl4. a thermal analysis was performed for this solution and it was found that its initial freezing point was – 28.7°c. a reliable source in the bibliography states that for ccl4, t°f = – 22.9°c, and its freezing point lowering constant is kf = 29.9°c/m. calculate the molar mass of the unknown solute.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, NorbxrtThaG
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
Asolution was prepared by dissolving 2.2 g of an unknown solute in 16.7 g of ccl4. a thermal analysi...
Questions in other subjects:

Spanish, 05.11.2020 22:00


Mathematics, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10


Mathematics, 05.11.2020 22:10

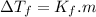
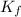 is cryogenoscopic constant of solvent.
is cryogenoscopic constant of solvent.