
Consider the following reaction: 2 no(g) + 2h2(g) → n2(g) + 2 h2o(g) the rate law for this reaction is first order in h2 and second order in no. what would happen to the rate if the initial concentration of no tripled while all other factors stayed the same? the rate will increase by a factor of 9. the rate will decrease by a factor of 3. the rate will double. the rate will triple. the rate will remain constant.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 09:20, nyceastcoast
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
Consider the following reaction: 2 no(g) + 2h2(g) → n2(g) + 2 h2o(g) the rate law for this reaction...
Questions in other subjects:



Chemistry, 04.12.2019 21:31







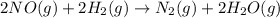
 = 2
= 2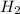 = 1
= 1![Rate=k[NO]^2[H_2]^1](/tpl/images/0229/1480/39530.png)
![Rate'=k[3\times NO]^2[H_2]^1](/tpl/images/0229/1480/e80a2.png)
![Rate'=k[3]^2[NO]^2[H_2]^1](/tpl/images/0229/1480/b88df.png)
![Rate'=k\times 9[NO]^2[H_2]^1](/tpl/images/0229/1480/d2dcb.png)




